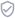
University Professor, Mohamed Abdel-fattah, admitted that he failed to declare £100,000 funding from mesh manufacturer.
Professor Abdel-fattah led the 2012 study that concluded that no patients suffered from thigh pain three years after mesh surgery.
In his orginal statement, he said he received £65,000 funding from The Henry Smith Charity - but failed to mention the other £100,000 he received from Coloplast - the manufacturer of a device used within the study.
After allegations of research misconduct, a correction to the study was published containing a link to the Professor's own declaration of interests. Thisincludes receiving money from various mesh manufacturers (including Ethicon and Coloplast) for being a consultant and trainer for mesh manufacturers.
What Should I Do if I Have an Implant?
If you are concerned about the safety of your mesh implant, the International Consortium of Investigative Journalists recommends that “your first point of call should be the medical team that performed the operation.
“If you cannot go back to them, you should consult your primary care doctor.”
You can also call the mesh counselling hotline on 0121 314 7075, Monday-Friday 8am-6pm if you have been affected by mesh implants.
What are Mesh Implants Used For?
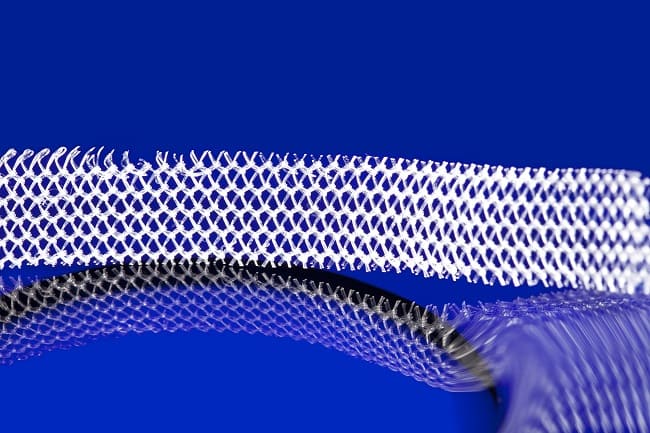
Mesh is a general term used to describe a variety of types of manufactured biological and synthetic implantable devices. It is used to support tissues in a number of surgical procedures. Most notably, mesh is used for surgical treatment of stress urinary incontinence and pelvic organ prolapse.
There are three main surgical procedures that can be performed to treat pelvic floor disorders:
- Transvaginal mesh for pelvic organ prolapse
- Transabdominal mesh for pelvic organ prolapse
- Mesh sling for stress urinary incontinence
What are the Complications of Mesh Implants?
For some women, mesh is an effective solution for treating pelvic floor disorders. However, some women also experience serious complications after enduring the procedure. Complications that can occur include:
- Mesh degradation – more surgery may be required to remove and replace the mesh
- Vaginal bleeding
- Vaginal discharge
- Damage to surrounding organs, e.g. bladder
- Infections
- Blood clot formation
- Further prolapse symptoms – requiring further surgery
Let us know your thoughts about the latest updates on mesh.
Previous Updates
Sources
[1] U.S Food and Drug Administration (2018) Urogynecologic Surgical Mesh Implants [online]. FDA [viewed 17/10/2018]. Available from https://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/urogynsurgicalmesh/
[2] Bladder & Bowel Community (2018) Vaginal Mesh Support [online]. Bladder and Bowel Support Company [viewed 17/10/2018]. Available from https://www.bladderandbowel.org/surgical-treatment/vaginal-mesh-support/
[3] BMJ 2018;363:k4164. Available from: https://www.bmj.com/content/363/bmj.k4164?fbclid=IwAR3hguEMJspK_MzsQiW8DpMukJ2QcfSFZseomKQzpEQgHm0CRmeQ5vVFU8w
[4] BMJ 2018;363:k4155. Available from: https://www.bmj.com/content/363/bmj.k4155
[5] BBC (2018) Medical device rules need 'drastic change' to protect patients [online]. BBC [viewed 26/11/2018]. Available from https://www.bbc.co.uk/news/health-46337937
[6] BBC (2019) Mesh Expert Failed to Declare £100,000 Funding [online]. BBC [viewed 24/01/2019]. Available from https://www.bbc.co.uk/news/uk-scotland-46972350?fbclid=IwAR2NkRO-QiuRcz__ohX3F72AUemjGVzXvEMvIDUW1C2Yf9vNqUzzHaf62W0